3D Bioprinters; Life From Scratch

Imagine this - you walk into a restaurant beneath a cool sunset evening and take a seat at the bar, after which you start looking through the thin menu that a passing waiter provided you with. Besides the usual meal categories and food labels that you've known this place to adopt (vegetarian, vegan, lactose-free, etc.), you then come across a 4 Oz beef steak in the third page of the booklet with a strange image next to it. Upon further investigation, you realise that this image - resembling a small sheet of squares, perhaps, or a printer - indicates that the steak did not come from a cow. Instead, it was the establishment's newly added prototype: a 3D-printed steak!
At the moment, there is no telling whether this item would taste anything like its butchered-cow alternative, nor if it would even be any good. (It also sounds a bit comical, I would say!) Nevertheless, this scenario is a lot more realistic than one might first think. Just last year, for example, Israeli company Meatech 3D revealed the first true-to-the-bone 4 Oz steak to have ever been 3D-printed. The main incentive behind this, as I understand it, is to create a more environmentally-friendly way of procuring meat than the tranditional method. No surprise, given that meat production is one of the most polluting and water-intensive industries around (with a poor tendency to spread animal cruelty through factory farming). As such, it is only a matter of time before food places all around the world start introducing their own printed edibles routinely.
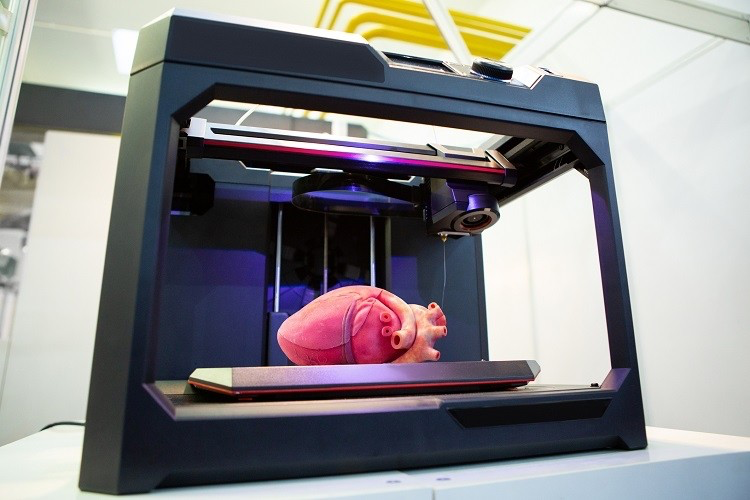
3D bioprinting is not limited to making foodstuffs, however. From drug development and dentistry to artificial organ synthesis, this technology is applicable to practically all fields that involve organic tissue. Being relatively recent, it is also liable to rapid improvement over time, especiallly with all the research on tissue regeneration in salamanders taking place right now. With all that said, let's take a look at what 3D bioprinting actually involves.
Programming Tissues
First arising as a combination of additive manufacturing (i.e., regular 3D printing) and biomedical engineering, 3D bioprinting utilises the precision of computer-aided design (CAD) and computer-aided manufacturing (CAM) to produce functional layers of either solvated biomolecules or cell tissue. While such has been attempted before with traditional tissue engineering techniques - wherein you'd grow a culture of cells on a platform and hope that they would form the structure that you want - this approach is both more efficient and less time-consuming than the alternative.
With 3D bioprinting, you can directly influence the placement of cells and their coordination with each other, harvesting cell cultures to add them to a scaffold biomaterial matrix (which we explore below) as a bioink. In this sense, you might be interested to know that 3D printing and bioprinting are really not much different to each other. Both depend on computer softwares for their printers to work, for one, progressively moving along a set path while stacking materials onto a plate below. Interestingly, both are further known to incorporate the same CAD softwares, including popular design engineering programs like Autodesk AutoCAD, SolidWorks and Pro/Engineer. Since most of these softwares are made to be user-friendly and easy to learn, a good number of biomedical researchers have already implemented them into their experiments. As an example, one 2018 study conducted by a team from Harvard Medical School demonstrated that TinkerCAD-assisted 3D bioprinting could be used to selectively add platelet-rich plasma (PRP) onto damaged tissue, generating growth factors (like the ones in plants) that could recover the tissue without causing an autoimmune response.
Of course, regenerative medicine is only the start of 3D bioprinting. It is also, I would say, one of its simpler methods of administration. The real challenge comes when you want to build a tissue from scratch, potentially allowing you to synthesise individual organs in the lab. Yet this is exactly what modern tissue engineering is all about - integrating current computer graphics to accurately construct biologically important 3D structures. Do note that the challenge doesn't rerally come from operating the computers, nor with the way that the printers are made. With the right technical skills, after all, the user can simply plop in some instructions and add bioink into the printer before it starts working fine. Indeed, the problem is not with the computers - it is with us (which seems to to be the case quite often nowadays, huh?).
All jokes aside, though, it is really only our understanding of the composition of organisms that limits the capabilities of 3D bioprinting. Yes, biochemistry research gives us much insight into the pathways that make up separate cells and tissues. In most multicellular organisms, we even know how the function of one cell or tissue or organ will influence another. Nonetheless, it is an entirely different matter to comprehend how even a single type of tissue develops to maturity, featuring the thousands of factors along the way (some of which come from other tissues and are needed only at certain times during maturation) that let it become part of a fully functional organism. So here's a question: how are we supposed to construct an organ if we do not understand all the minute steps of how it is made naturally? 'By trial and error,' is my answer, starting with a more basic structure as a test.
Fortunately, the knowledge that we do have allows us to roughly estimate how to do this. Let's take the beef steak from before. To begin with, we know that cow muscle mainly contains a mixture of muscle fibres, adipose tissue, cardiovascular cells in the form of blood vessels and the protein-secreting fibroblasts that produce the extracellular matrix (with proteins like collagen fibres giving it strength and stability). By repeatedly culturing these cells individually (or, rather, culturing their respective stem cells) and mixing them in known fractions with a scaffold material, we can then create a bioink that is stacked on top of itself to produce the beef's base. After each layer, we might also change the material fractions in the bioink to more accurately mimic how muscle tissue changes in a cow - though it would probably be better to stick with one bioink for the steak.

If the printed tissue displays regular function, then congratulations! You've succeeded in making a copy of bovine muscle tissue. If not, then it is only a matter of improving your construct by altering the cell ratios in your bioink and perhaps the environment on which you print them. I should mention that this includes the scaffold material that you use; whether it is made of hydrogel, nanomaterial, or an acellular dermal matrix (ADM) for skin tissue prints, each scaffold will be more suitable for different types of bioink. Nevertheless, once you've got the recipe down, it should in theory be easily reproducible and maintainable. Due to the layer-by-layer construction of the tissue, it possesses interconnected pores that allow fluids to flow freely throughout the structure, making it ideal for the spread of nutrients, growth factors and signalling molecules between cells.
So, now that we have an idea of what 3D bioprinting is all about, we come onto the proverbial icing of the cake: the printers.
Printing Life
There are four main 3D bioprinting techniques commonly used in the lab, and each kind features its own distinct set of pros and cons. The first type, inkjet bioprinting, is the most popular because of its low cost and large availability world-wide. As with regular 3D printing, inkjet printers work by applying pressure to spew bioink through their nuzzle, doing so either in a continuous (CIJ) or drop-on-demand (DOD) fashion. The difference between these is only when they eject ink drops; whereas CIJ printers do so non-stop and recyle drops back into the storage when they are not supposed to be ejected (according to the design of the print), DOD printing ejects drops only when needed. Consequently, DOD inkject printers are usually preferred for tissue engineering, as they do not have to recycle their bioink and so have a lower risk of contamination.
Typically, inkjet printers are fitted with a heater that vaporises droplets of bioink in a microfluidic chamber with a powered current, which in turn pushes out the bioink below by the pressure generated from the bioink bubble. These thermal inkjet bioprinters are modified to produce just enough heat to print, while still staying cool enough to not damage the cells in the mixture. Of course, there are still exceptions to this rule in biology, so sometimes we need to use something more appropriate. In this case, we might instead use a pizoelectric inkjet bioprinter. Utilising a super fancy little gadget known as a pizoelectric transducer, this machine basically converts the electric field generated by an associated current into a force, hence avoiding any temperature fluctuations in the system. This is more expensive, however, so pizoelectric printers are generally seen only in larger or more specialised facilities.
Then we have extrusion bioprinters, which also put pressure on bioink in a chamber but do so more powerfully. If inkjet printers are the carpenters of biomedical engineering, then these are the drillers and excavators - bulky, heavy-weight and packing one hell of a punch. They can be piston-operated or pneumatic (where air is sucked into the chamber to create pressure), though both usually lack precision and can lead to a large portion of bioink cells getting damaged. As such, extrusion bioprinters are mostly utilised when the bioink to be dispensed is more viscous and dense than other printers can manage. Fortunately, they are still relatively inexpensive, so they always remain a viable option.
Laser-assisted bioprinters (LABs) are more complex, but they essentially direct a focused laser beam onto a metal film-protected layer of bioink, transferring sufficient energy to displace a droplet from the ribbon. As the bioink needs time to mix back together before it can be displaced again, the laser comes in pulses only microseconds apart - all of which makes LABs very slow and expensive pieces of technology. These are still worth it, however. Causing the least harm on cells and biological material, LABs keep over 95 percent of stored bioink intact. They also display an incredibly high accuracy and precision, and are even able to dispense high-viscosity bioinks like the aforementioned extrusion printers.
Finally, there are stereolithographic bioprinters, which in my opinion are the coolest of the lot. Focusing on the vertical aspect of a model, these begin by placing a platform at the surface of a photo-sensitive/heat-curable bioink, thereby moving it upwards over time while light is projected at the exact positions that are to be printed (as instructed by the computer graphic). Plane by plane, the bioink solidifies into a 3D structure - and it does so from top to bottom! While not quite as precise as LABs, these probably have some of the greatest potential for organ synthesis, so I expect they'll receive a fair amount of attention in the coming years.
Of course, all of these printers are exceptional forms of technology in their own right, and each serves its own purpose in the world of research and biomedicine. As such, I would personally be quite happy working with any one of them someday - because this field really is quite exciting, don't you think?
References
- Agarwal, S. et al (2020). Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–A Review. Frontiers in Mechanical Engineering 30. Retrieved from https://doi.org/10.3389/fmech.2020.589171
- Pakhomova, C., Popov, D., Maltsev, E., Akhatov, I. & Pasko, A. (2020). Software for Bioprinting. International journal of bioprinting 6(3):279. Retrieved from https://doi.org/10.18063/ijb.v6i3.279
- Faramarzi, N. et al (2018). Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds. Advanced Healthcare Materials 7(11):e1701347. Retrieved from https://doi.org/10.1002/adhm.201701347
- Vanaei, S. et al (2021). An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Engineered Regeneration 2:1-18. Retrieved from https://doi.org/10.1016/j.engreg.2020.12.001
- Knowlton, S., Anand, S., Shah, T. & Tasoglu, S. (2018). Bioprinting for neural tissue engineering. Trends in Neurosciences 41(1):31–46. Retrieved from https://doi.org/10.1016/j.tins.2017.11.001
