Building Bubbles: Clathrin-Mediated Endocytosis

In all forms of organised systems, communication and transport go hand-in-hand. Human civilisation itself displays this in a myriad instances. When we need a quick meal at home, we might call the local Thai restaurant to get a tasty takeaway curry sent to us. Likewise, sending a package to a faraway friend might take you a trip to the mail services, where you'd thereby relay the request to the attendant before the package is sent.
Naturally, speaking is also a way of communication. If one delves even deeper into the issue, however, one might even argue that it is a form of transport - with energy being transferred from one person to another via the longitudinal particle vibrations in sound waves. Cells, too, have their own abstract relay pathways. They don't use sound for this, however; they use proteins.
Although cells are living beings, they are also machines without a consciousness. Transporting nutrients or signals and communicating with each other (or with themselves) can therefore be somewhat unintuituve at first glance, particularly since different cell types have their own communication mechanisms (like quorum sensing in bacteria). As complex as they might be, however, puzzling out these pathways is key to the advancements of medical treatments and drug discovery. Clathrin-mediated endocytosis is one such pathway - and it displays some of the most convoluted and fascinating protein chemistry we know.
Cell Absorption
As a general term, endocytosis refers to how cells absorb substances in their external environment, packaging them inside vesicles to be transported around the cytoplasm. As this involves crossing the plasma membrane of the cell - an envelope constituted mostly of fats called phospholipids in a bilayer - it also tells us that any material endocytosed is polar and hydrophilic. Both of these terms are interchangeable, and they basically describe a molecule that is water-soluble and so cannot dissolve in fats. Small molecules like oxygen, carbon dioxide and carbon monoxide are all non-polar, so they are lipid-soluble and can freely bypass the membrane (in a process known as simple diffusion). Comparatively, the majority of large molecules in the body are polar, making it necessary to have specialised transport systems for getting them into and out of the cell.
Note: endocytosis is not the only way polar molecules can enter a cell. Dedicated protein channels and transporters also allow this via facilitated diffusion, and are really the preferred manner of material uptake. This is especially true when you realise how efficient they can be in comparison. While one cycle of endocytosis typically takes substantial amounts of time and energy to absorb a couple thousand molecules at a time (as we explore below), some protein channels can carry through over a billion molecules per second...without even consuming any extra energy!
So why do cells have so many absorption pathways? In the same way that we use different vehicles for different jobs, it is all to do with what the molecule transported is required for. Let's take low-density lipoproteins (LDLs) as an example. These are large globules made mostly of fat with small quantities of embedded proteins, and they transport the nutrients around the bloodstream to deliver them to cells around the body. Nicknamed the 'bad cholesterol' of the body, LDLs are also the main cause of atherosclerosis (i.e., the clogging-up of blood vessels) - so it is often in our interest to decrease LDL levels in our blood.
Fortunately, when given enough time, cells lower LDLs for us. Specialised LDL receptors on the plasma membrane bind to proteins on LDLs passing by, which in turn causes the membrane to invaginate and form a pit. Through the cumulative action of various proteins in the cytoplasm (in this case including clathrin), the membrane wraps all the way around the LDL, till it eventually connects with itself and separates from the plasma membrane to form a clathrin-coated vesicle (CCV). Afterwards, the CCV goes on to fuse with an endosome in the cell (essentially an early storage vesicle for endocytosed material), which finally breaks it down with digestive enzymes as the endosome matures into a lyzosome. The remaining fats and proteins from the LDL can then be used by the cell for other purposes.
I just described the entirety of clathrin-mediated endocytosis, by the way (albeit rather simply). Altogether, this should give you an idea for why such methods of transport are sometimes used instead of the average protein transporter. Compared to the general gate-like mechanism of facilitated diffusion, endocytosis allows cells to more efficiently sort and direct molecules. Not to mention, the vesicles involved give cells a wonderful way to defend themselves from toxic substances, keeping them in tidy little bubbles before they can damage any important machinery in the surroundings. Vesicular trafficking, as it is called, is crucial for the health and survival of a cell.
In reality, endocytic mechanisms make up a diverse family of metabolic pathways. They include pinocytosis for water and mineral uptake; caveolar endocytosis, which is entirely independent of clathrin proteins; and phagocytosis, an energetically demanding procedure associated with certain types of immune cells and used to trap pathogens invading the body. All are important in their own respects and at the appropriate times. Nevertheless, their clathrin-dependent counterparts are the most commonly found in cells - not only in us, but rather in all eukaryotes on Earth.

Protein Layers
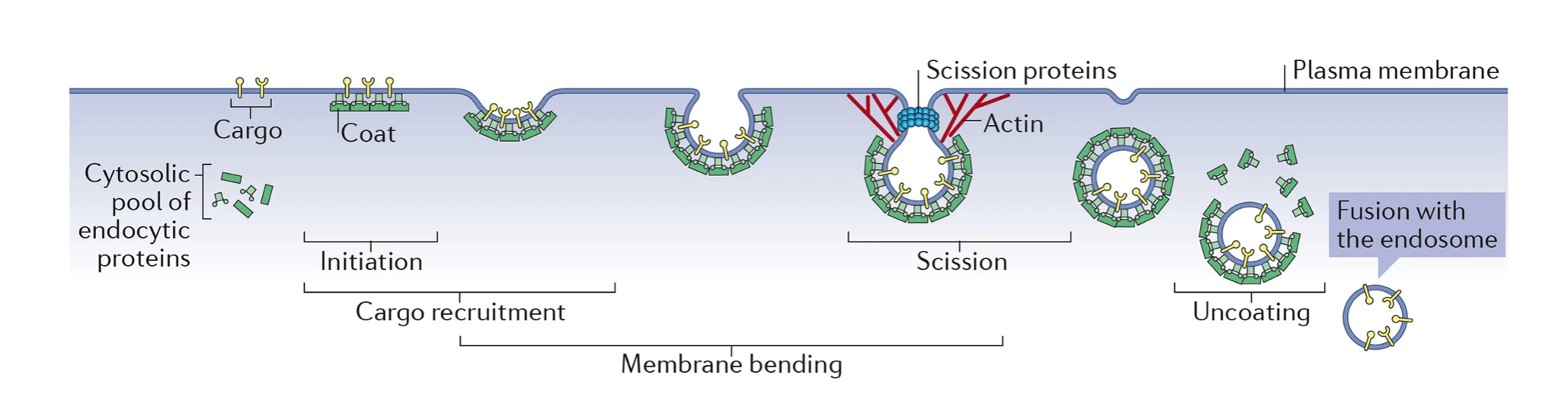
In practice, clathrin-mediated endocytosis (which I will refer to as 'endocytosis' from here on out for the sake of simplicity) begins via the interaction of a number of signalling molecules with transmembrane protein receptors in the plasma membrane. Such binding events trigger a conformational change in the receptors - wherein their structures morph to more 'comfortably' fit the bound molecule - and consequently alter their shape at the cytoplasmic side of the cell. At this point, the signal-receptor complexes are known as the cargo, and it is the cell's job to concentrate them into one area before a clathrin-coated pit can form.
Interestingly, while the initiation of endocytosis can occur randomly by the stochastic collisions of endocytic proteins, it is normally observed to be spatially non-random. In other words, cells contain certain components that repeatedly form endocytic sites at the same locations, again demonstrating a directionality in vesicular trafficking. Currently, though, we are not sure whether the cargo plays an essential role in this. It is likely that the local differences in cargo concentrations (due to their polymerisation when near each other, perhaps) and plasma membrane phospholipids have a combined effect on endocytic initiation, but further research must be carried out to ascertain this.
During the formation of an endocytic site, protein adaptors in the cytosol (the liquid part of the cytoplasm) join to phospholipids, with scaffold proteins stacking on top to create what is sometimes referred to as the pioneer module. In mammalian cells, this is thought to be made at the least of the adaptors FCHO1/2 and AP2, as well as the scaffold proteins EPS15, EPS15R, intersectin 1 and intersectin 2. Since a few of these (FCHO1, AP2 and EPS15) bind directly to cargo and most bind to specific types of phospholipids in the plasma membrane, one theory states that such pioneer module proteins must first exceed a minimum threshold concentration before endocytosis can proceed. To me, this makes a lot of sense. Forming a CCV requires a bunch of chemical energy, both to create the proteins necessary for it and to then use them to apply mechanical pressure on the membrane - so preventing endocytosis from taking place when it's not worth it seems pretty logical. But I digress.
Now, I mentioned some 'specific types of phospholipids'. Just to expand on that a bit, these are actually a large family of lipid anchors in the membrane called phosphotidylinositols (PIs). When a phosphoryl group is added to them (via phosphorylation), these are then known as PIPs, or phosphoinositides. As with seemingly all biochemical pathways, the conversion of PIs into PIPs and vice versa involves a wide range of enzymes, each regulated by intramolecular signals that adapt the cell to its environment. In terms of endocytosis, however, all we need to know is that PIPs are up-regulated at the endocytic site in order to hold the adaptors in place.
Once the pioneer module is constructed (with each AP2 protein anchored to both the cargo and PIPs), the adaptors and scaffold proteins interact with each other to stabilise the structure. Intersectins 1 and 2, for example, have previously been shown to possess domains complementary to other endocytic peptides. Such interactions allow precise positioning of adaptors around the pit being made, and in total can stimulate a more rapid addition of endocytic machinery. As further proteins are placed around the membrane (e.g., the adaptor Dab2), the main player finally begins to make its appearance. Introducing: clathrin triskelions. As the name suggests, these trimeric complexes are made of three clathrin molecules each, hence featuring three curved arms around a centre. On a picture, they even look somewhat like starfish! (They're not starfish, though.)
Clathrin Polygons
After contacting PIPs, cargo and other neighbouring proteins, adaptors like AP2 and Dab2 experience a conformational change that allows clathrin to join them. In turn, clathrin triskelia are able to form a final layer around the pit - and it is this that produces most of the mechanical force needed to induce curvature in the membrane. The most popular model at the moment for clathrin coat-mediated bending, by the way, is that it assembles over time onto a pit of constant curvature. This means that the radius of the pit doesn't really change after initiation, with the CCV at the end having the same radius as its predecessor.
(Apparently, actin polymerisation is also vital in membrane bending, with intersectin domains and other proteins recruiting nucleation promoting factors that catalyse it. This is particularly important in yeast cells and other organisms with high internal osmotic pressures, as here clathrin triskelia would not be able to bend the membrane by themselves.)
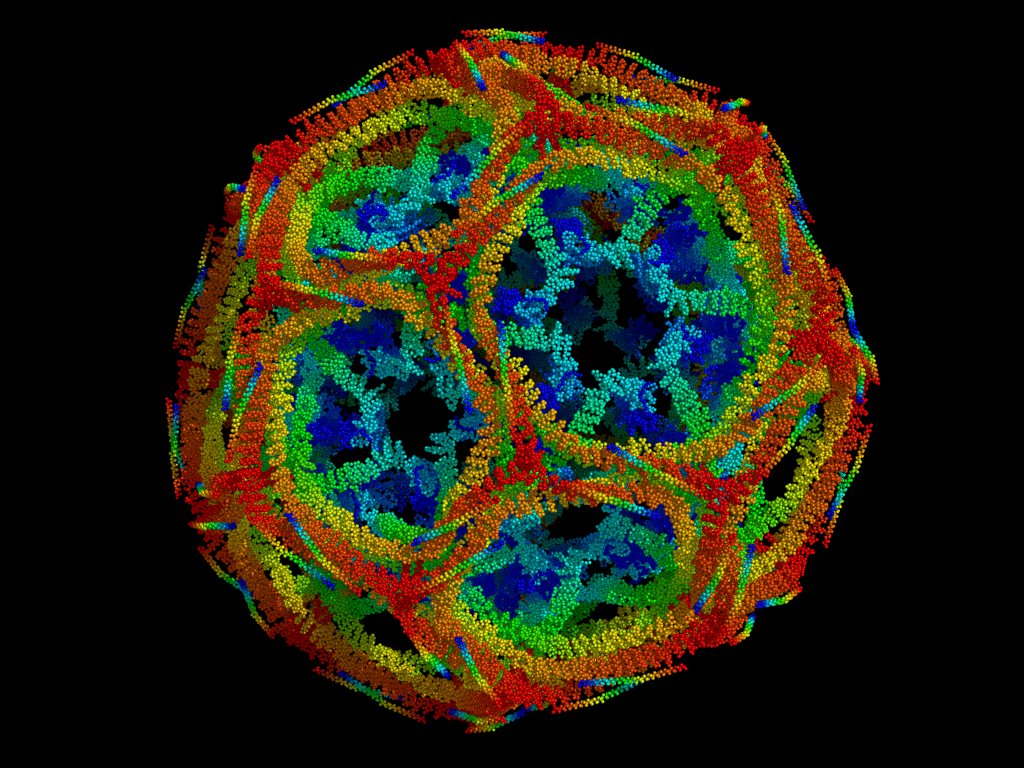
Rather fascinatingly, clathrin triskelia are regularly interspaced around the vesicle so as to build what appears to be a net. By using cryo-electron microscopy, researchers have found that clathrin 'nets' form hexagons between the triskelia, with pentagons spread about to make it curved. Looking a bit like a football (and also like coccolith shells), the nets are mostly invariable in their polygonal composition. They always need to have at least 12 pentagons, for instance. As such, we find that the smallest nets (so-called 'mini-coats') depict 12 pentagons linked by 4 hexagons. The prototypical clathrin coat comprises 12 pentagons and 20 hexagons, and so is given the absolutely marvellous name of a truncated icosahedron.
Finally, the cargo-bearing, protein-layered CCV gets pinched off the plasma membrane by the action of dynamin. Held by intersectins, dynamin twists around the membrane tubule - the remaining link between the vesicle and the plasma membrane - and constricts. This is powered by GTP hydrolysis, and is followed up by the ultimate disassembly of the coat in the cytoplasm. The cell doesn't need these proteins to transport vesicles, after all; it only needs them to create them. Instead, cells use a whole other family of proteins for transport - but that's probably a topic for another time!
References
- Kaksonen, M. & Roux, A. (2018). Mechanisms of clathrin-mediated endocytosis. Nature Reviews Molecular Cell Biology 19:313–326. Retrieved from https://doi.org/10.1038/nrm.2017.132
- Sorrentino, V. et al (2013). The LXR-IDOL axis defines a clathrin-, caveolae-, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation. Journal of Lipid Research 54(8):2174-2184. Retrieved from https://doi.org/10.1194/jlr.m037713
- Mettlen, M. et al (2010). Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. Journal of Cell Biology 188(6):919–933. Retrieved from https://doi.org/10.1083/jcb.200908078
- Lieber, A. D. et al (2013). Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Current Biology 23(15):1409-17. Retrieved from https://doi.org/10.1016/j.cub.2013.05.063
- Pérez-Gómez, J. & Moore, I. (2007). Plant Endocytosis: It Is Clathrin after All. Current Biology 17(6):R217-R219. Retrieved from https://doi.org/10.1016/j.cub.2007.01.045
