The Alchemy Of Today: Turning Water Into A Metal

Every year, research journals publish thousands of articles worldwide, but only a select few can truly take the scientific community by storm. In regards to the physical sciences, one only has to look so far as Claude E. Shannon's 'A Mathematical Theory of Communication' from 1948 - which very well might have been the catalyst to creating the internet - and perhaps the papers that led to the discovery of semi-conductors to see how such papers can change the world. Nowadays, the accelerated development of cutting-edge technology means that we see such game-changers almost yearly. In fact, just recently in the summer of 2021 (the current year at the time of writing), we were able to see one of the most bizarre spectacles proven experimentally to date. Like the apocryphal alchemists of old, scientists had turned water into a metal.
Alchemy and the transmutation of elements has always had a part in our society. Even today we see inspiration taken from the concept in our arts and entertainment, and there are probably even a couple of people who genuinely believe in the prospect of alchemy today. It is not all vague stories and magic, however, so they may have a point. In reality, all elements are now well-known to be made of tiny particles called atoms (which possess a rather interesting history of how research led to their discovery). Atoms, in turn, are composed of even smaller subatomic particles (such as electrons and quarks) that are held together by nuclear and electrostatic interactions. This is the case for all elements in the universe that we know of.
To take an example, sodium and magnesium atoms are only distinguished by how the latter contains one more proton and electron than sodium. In fact, under the right circumstances, you could turn practically any atomic element into any other type of matter you wanted; and that most certainly includes turning lead into gold. Alas, there is an emphasis on 'under the right circumstances,' as often these are quite tricky to reproduce. To be exact, you would generally require approximately the same pressure and heat as what is found at the centre of our sun - and even then it would only be sufficient for the simplest of transformations (i.e., hydrogen to helium). While this is still technically possible to do in the lab, it is definitely far from being commercially feasible.
Albeit, Pavel Jungwirth and his team from the Czech Academy of Sciences, Prague, did not make use of high temperatures nor high pressures to create metallic water. Their idea was a simple one - but it was abstract enough that nobody had thought of it before. So what exactly did they do?
The Charged Structure Of Metals
To understand how water can be turned into a metal, we must first be clear on what differentiates a metal from a non-metal in the first place. As aforementioned, all elements - from oxygen to iron to uranium - are composed from the same subatomic particles, with each element always having a specific number of protons in its nucleus (e.g., oxygen atoms always contain 8 protons each). When uncharged, atoms also have an equal number of electrons surrounding their nucleus. Likewise, adding or taking away electrons from an atom makes it charged and turns it into what is known as an ion. Positively charged ions are in turn labelled cations, with anions being negatively charged ions.
In essence, metals are defined structures made entirely of cations, with electrons (previously bound to their respective atoms) moving freely and delocalised around them. Since electrons are negatively charged, this means that they form attractive electrostatic interactions with the surrounding cations and hold them strongly in place, forming a highly stable structure known as a metallic lattice. This is why many metals only melt at temperatures present only in volcanoes or blast furnaces: a lot of energy is required to break the electrostatic attractions inside them. It should also be mentioned that electrons only tend to delocalise from atoms that are more stable when positively charged than neutral - a property that just so happens to occur in elements like sodium, potassium, iron, etc. (See the octet rule and ionisation energies in metals for more details.)
Non-metals, of course, do not bear this property. Most of the time, they exist as covalent molecules that share electrons between neutral atoms (as with diamond or silica), or sometimes salts that contain cations and anions rigidly interlocked with each other. There is a list of subsequent chemical properties associated with metals versus non-metals, but I won't go into them here. Just know that, as a covalent non-metal, water does not possess any charged particles floating around normally. (As a side note, this also means that pure water is actually an excellent electrical insulator and cannot conduct electrical currents by itself. It is instead the dissolved charged ions that come from mineral impurities that allow it.)
To change a non-metal like water into a metal, scientists originally thought that you needed to essentially force the material's molecules to come so close together that they would be more energetically stable by losing electrons to form a metallic lattice with them. While this sounds fairly straightforward, physicists, being the maths enthusiasts that they are, have previously calculated that the pressures needed to make water metallic would have to be in the range of 50 million times the earth's normal atmospheric pressure at sea level. In other words, we would require the pressure supposedly experienced at the centre of Saturn. Fortunately, we just recently found that that is not necessary in the slightest.
A Transient Transmutation
So, back to the team in Prague. Needless to say, their method for turning water into a metal was unconventional. Instead of brute-forcing water molecules to enter that state with pressure, they thought, why not just infuse them with so many outside electrons that they become ionic by themselves? Again, though previously overlooked, the idea was simple - but it is often the simplest ideas that tend to work the best.
Using an alloy (i.e., a mixture of metals) of pure sodium and potassium that is liquid at room temperature, the team filled a custom dropper with a micronozzle that led into a small vacuum chamber. Here, instead of increasing the pressure, they decreased it to be only about 10 millionths of the standard atmospheric pressure. (Certainly much lower than the pressure in Saturn!) Furthermore, the team took arduous efforts to minimise any impurities in the vacuum chamber so that it would only contain gaseous water molecules, i.e., vapour. The consequent experiment consisted simply of repeatedly adding alloy drops to the chamber and altering the pressure accordingly. After much trial-and-error, the team ultimately observed a shiny, gold-coloured layer forming over their drops, which over a few seconds turned into a spectrum of wondrous blues, purples and silver. Though the images produced were admittedly not of the highest quality, they were still fascinating to watch (both in real life and in pictures!).
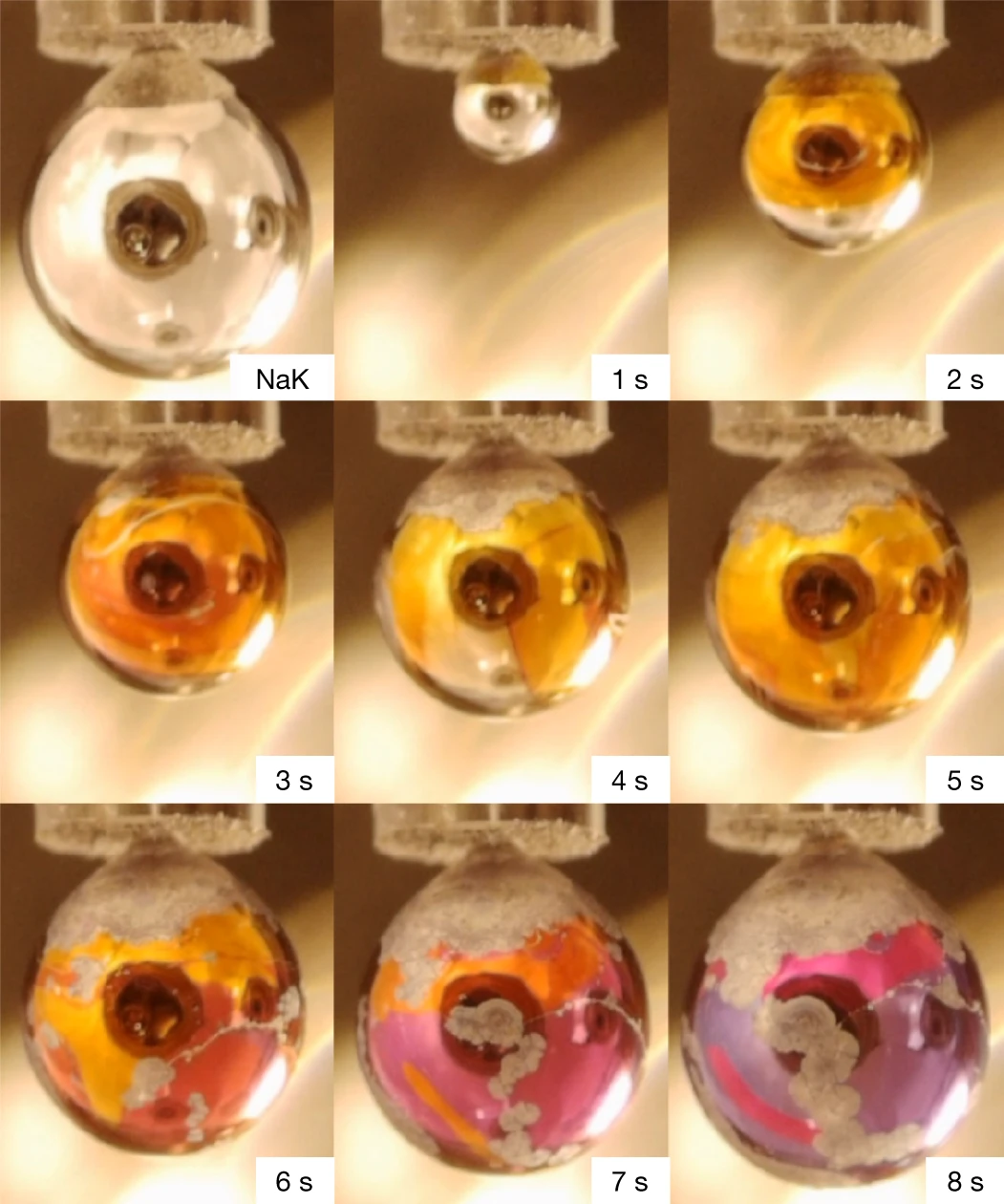
The golden layer was, of course, metallic water. So what happened? Simply put, since sodium and potassium are both alkali metals - which means that they ionise easily and have sufficiently low melting points to form the required liquid alloy - they produced cations and delocalised electrons very stably. When in the vacuum chamber, these were then dissolved in the coating of water molecules to create an electron density of 5 × 1021 electrons per cubic centimetre (which was incredibly high for the number of water molecules that were adsorbed to each drop). Normally, when you mix water and an alkali metal together, they react rapidly and violently without producing the sort of metallic layer that Jungwirth saw. This is due to the chain reaction generated by the spontaneous reactions of individual molecules, emitting enough energy for neighbouring molecules to react (which is what we observe when wood and other organic fuels combust). And this is why the researchers lowered the pressure so much; the water molecules were so few and far between that they allowed the metal ions and electrons to dissolve without reacting with them - or not very quickly, at least.
After a couple of seconds of staying intact, the authors noted that the water would eventually react completely with the metals to make hydroxide and hydrogen ions. After this point, the metallic water would be lost. Even this short period of characterisation was more than was expected, however, as other calculations (again involving the pressure found in large planets) predicted that metallic water could only exist in the lab for a maximum of a few milliseconds. Instead, the metals-turned-electrolytes continued floating about the water for an observed maximum of approximately 8 seconds - which is thousands of times longer than predicted. Not a bad outcome, I would think!
With the experiment in mind, we can see why the kind of metallic water that the authors created was not, strictly speaking, made purely of water. Indeed, the 'metallic' part of the water was actually induced by metal cations and electrons dissolved in it, resulting in the metallic effect that was thereafter characterised using X-ray photoelectron spectroscopy (an experimental technique that proved that the images were displaying metallic water as they described, involving the forced emission of electrons from the tested object to see how its energy levels would match with predetermined data). Furthermore, the same authors had previously obtained the same results with liquid ammonia, using a similar experimental methodology to turn it metallic (though without the need of a vacuum chamber). Albeit, it is true that nobody has ever created or witnessed the same phenomenon with water before, and so my hat goes off to them. In an explosive age of outstanding research and furious communal competition, they were able to make the academic community 'raise a 'brow,' per se. And theirs, I think, was definitely a rather wonderfully curious investigation.
References
- Mason, P.E., et al (2021). Spectroscopic evidence for a gold-coloured metallic water solution. Nature 595:673–676. Retrieved from https://doi.org/10.1038/s41586-021-03646-5
- Hermann, A., Ashcroft, N. W. & Hoffmann, R. (2012). High pressure ices. PNAS 109(3):745-750. Retrieved from https://doi.org/10.1073/pnas.1118694109
- Smith, R., et al (2014). Ramp compression of diamond to five terapascals. Nature 511:330–333. Retrieved from https://doi.org/10.1038/nature13526
- Buttersack, T., et al (2020). Photoelectron spectra of alkali metal-ammonia microjets: From blue electrolyte to bronze metal. Science 368(6495):1086-1091. Retrieved from https://doi.org/10.1126/science.aaz7607
